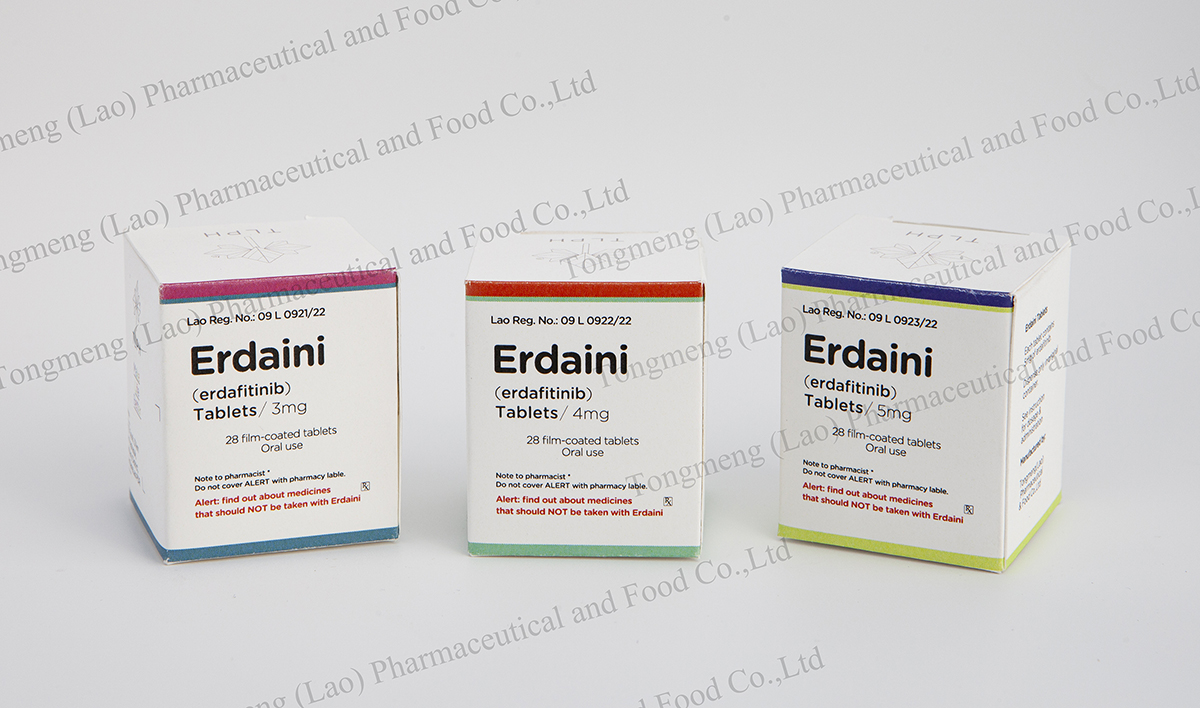
E-mail: info@tlph.com.la

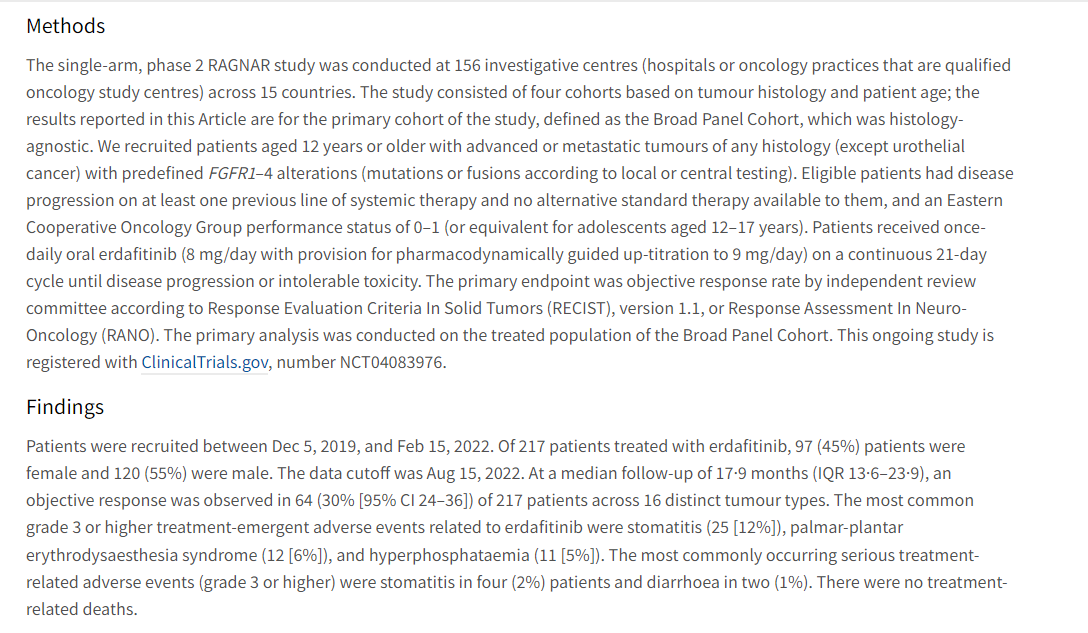
An international, single-arm, phase 2 study (RAGNAR) focus on Erdafitinib in patients with advanced solid tumours with FGFR alterations. The data cutoff was Aug 15, 2022. At a median follow-up of 17·9 months (IQR 13·6–23·9), an objective response (ORR) was observed in 64 (30% [95% CI 24–36]) of 217 patients across 16 distinct tumour types.

The clinical study RAGNAR results show clinical benefit for erdafitinib in the tumour-agnostic setting in patients with advanced solid tumours with susceptible FGFR alterations who have exhausted other treatment options.